When We Talk About Diamond Color
Natural diamonds are formed deep within the Earth under the extreme heat and pressure. Due to this process of creation, diamonds will carry a unique chemical structure based on the minerals present. Often, the minerals present during the formation process impacts the presence of color in the rough diamond material. Determining a diamond’s color grade is objectively grading the absence of naturally occurring color. A chemically pure and structurally perfect diamond will be completely colorless, like a pure, unadulterated drop of water. The purity of material is rare, which is why diamonds with high color grades are tremendously valued.
GIA Standards:
When GIA developed the 4Cs of diamond grading in the 1940s and 50s, its aim was to create an objective and standardized language to be used when describing the characteristics of a diamond. Because the diamond market is global, different centers of the trade around the world used different terminology, which could easily be confused or misinterpreted.
These scales identified the absence of color in a variety of ways which could be inconsistent and misunderstood. One commonly used grading method had three color grades - A, B, C. In order to establish a new color grading system with more room for the varieties of subtle color differences, GIA started their scale with D as the highest color grade and went all the way to Z. Once the Gemological Institute of America implemented the 4Cs of diamond grading, it became a universal standard of assessing color and few people wanted to cling to an outdated method of grading
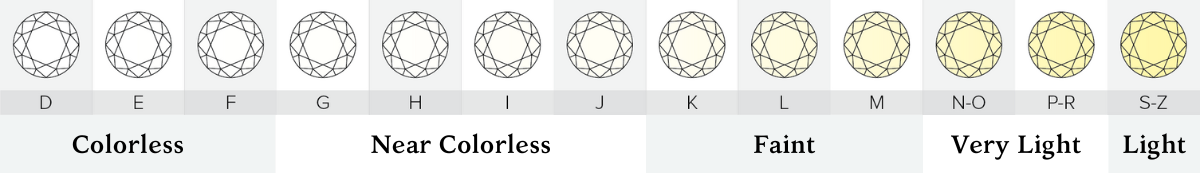
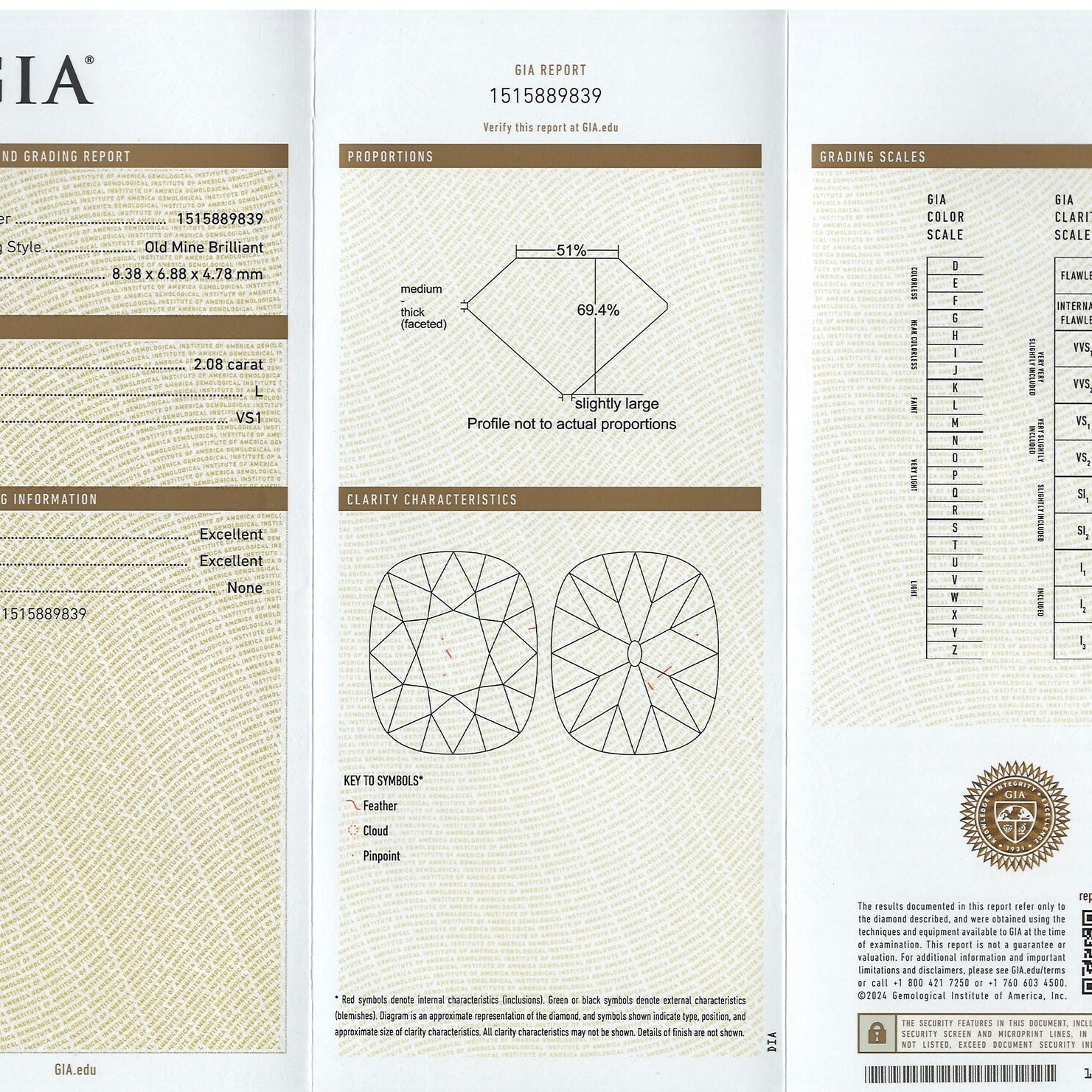
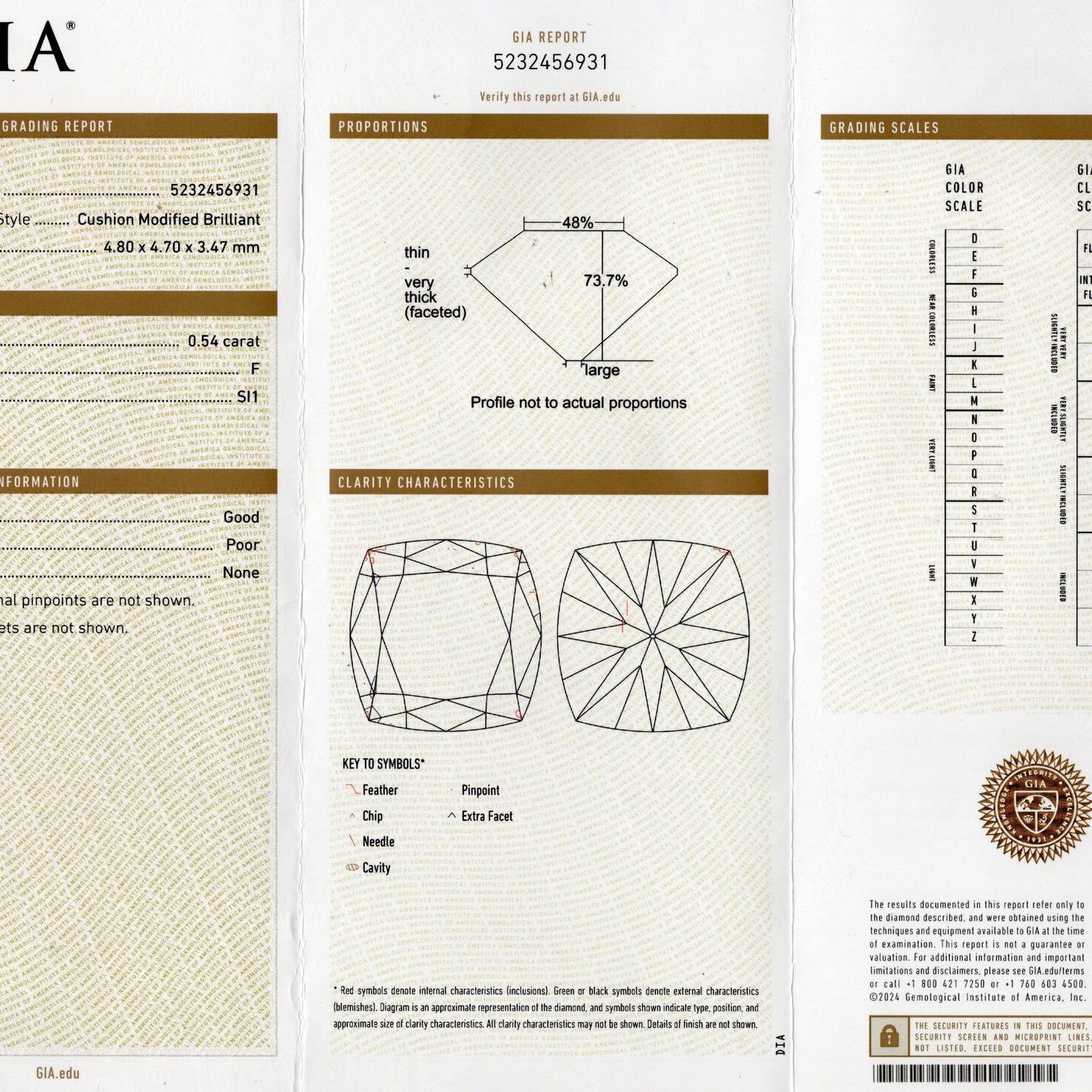
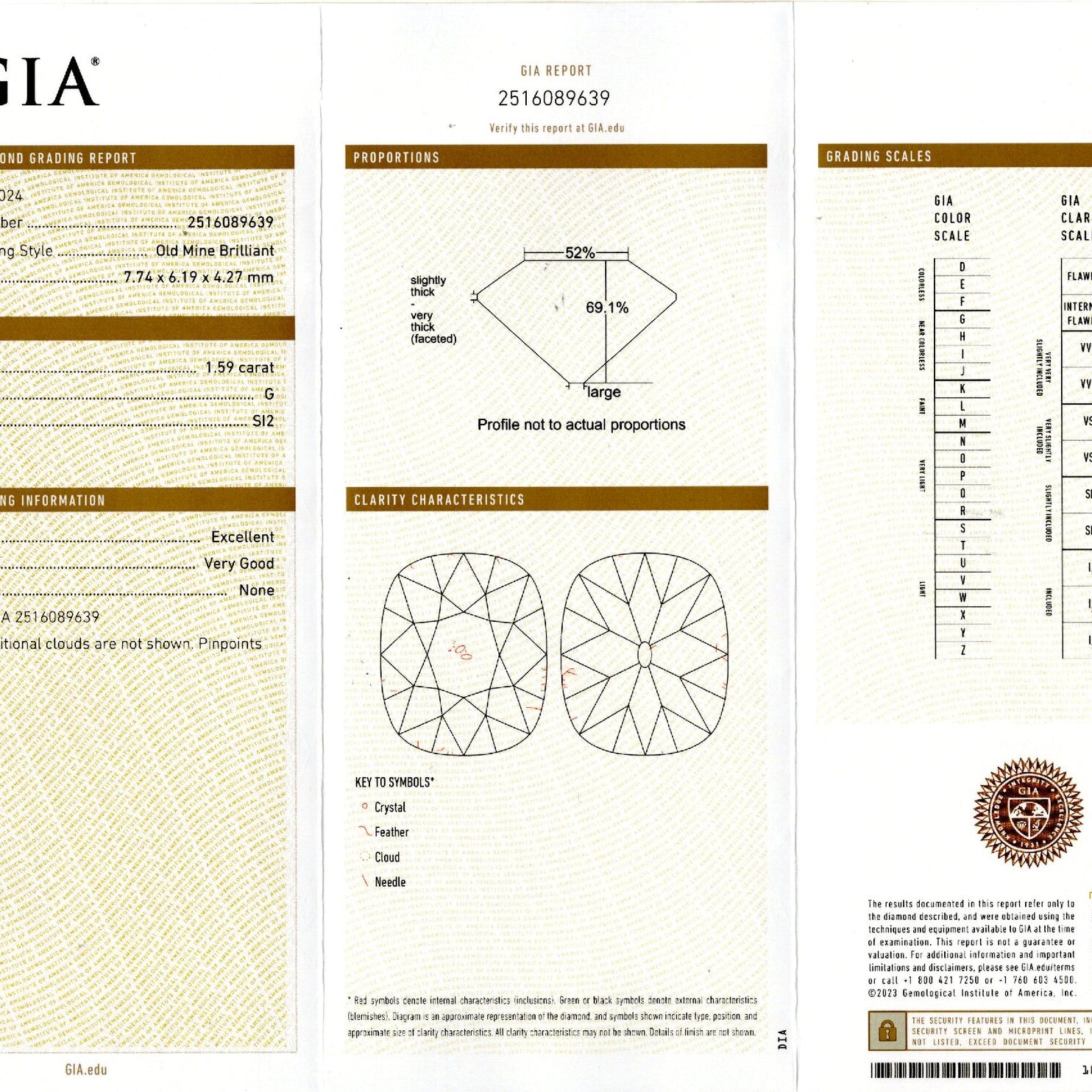
The GIA diamond color scale has 23 degrees of color from D-to-Z. The scale starts with D (perfectly colorless) and proceeds through the alphabet to Z (diamonds will show light brown, light yellow or light gray color).
On the GIA color scale, the letter grades are grouped into categories of colorlessness:
Colorless (D E F) - The most chemically pure diamonds of the D-to-Z scale.
Near Colorless (G H I J) - Color is often not noticeable except by trained diamond graders.
Faint (K L M) - Color is still difficult to see by the untrained eye.
Very Light (N-R) - Color can be seen in larger stones by the untrained eye.
Light (S-Z) - Color can be seen in many sizes of stones. Diamonds appear distinctly yellow, brown or gray, but not so saturated to be considered a fancy colored diamond.
How Shape and Cut Affects Color Appearance
When you're considering what color grade to look for, one aspect to consider is the shape and cut of the diamond. Fancy shapes tend to show more color than round diamonds of the same grade. This is especially true for elongated shapes.
For example, take a look at the G and I color round diamonds, and then compare to the G color radiant. In my opinion, the I color round looks comparable in color to the G radiant, while the G round looks much brighter white.
Why is this happening? Without getting too technical, it's the sparkle! Diamonds are graded for color by looking at them from the side. That way you're getting a true view of the body color of the stone. But when you turn the diamond face up, then you're getting white light bouncing around and reflecting off the facets. The particular brilliance of a round keeps it appearing a brighter white.
How Fluorescence Affects Color Appearance
Fluorescence in diamonds is a common occurrence and considered an identifying characteristic. The presence of fluorescence in a diamond does not affect its color grade, however it can impact the way a color appears in a natural diamond. Fluorescence refers to the strength or intensity of reactivity to longwave UV, an essential component of daylight, and is described as None, Faint, Light, Strong or Very Strong on a diamond grading certificate. Blue fluorescence is most common, but other colors are possible as well.
Under bright sunlight, a diamond with stronger blue fluorescence can appear whiter than its color grade might suggest. However, under the same lighting, diamonds with Very Strong fluorescence may have an oily or hazy appearance - a characteristic that would impact a diamond's ability to reflect light and sparkle.
This is a perfect example of how you shouldn’t judge a diamond by its certificate! Often, a diamond may seem like it is of low quality simply based on the color and/or clarity grade determined by a grading laboratory. However, when a diamond is viewed in person - especially in natural daylight conditions - YOU can be the judge of its beauty! For example, a great way to find a beautiful diamond with a bright white appearance is to seek out a stone that is graded lower on the color scale and has stronger fluorescence. Afterall, will you be admiring the diamond or the certificate?
Color Enhancements
Diamond manufacturers may treat their materials of lower color grades in order to “improve” the natural characteristic of the diamond material or to enhance the appearance and saleability of these diamonds. This can be a cost-effective way for a consumer to acquire a diamond with bold color that could be next-to-impossible to find in nature. Due to the fact that diamonds are both expensive and highly sentimental products, it is important for any treatment to be disclosed upon purchase so that a thing of great importance and value is not misrepresented. Additionally, the methods which create these extraordinary colors can be at risk of losing or altering these colors during routine maintenance, so it is always important to disclose any known treatments to your jeweler as well.
High-Pressure High-Temperature (HPHT) treatment is one effective method of changing the color of a diamond. This treatment method imitates the extreme heat and pressure process of natural diamond formation. Controlled exposure of diamonds in HPHT treatment can lighten the color of some brownish diamonds, enhance the color of natural yellow diamonds and also create certain fancy colors.
Low-Pressure High Temperature treatment is another method which mimics the circumstances of natural diamond formation within the Earth. This annealing treatment causes graphite to form in surface-reaching fractures. This method has become a standard treatment in achieving black diamonds, so much that it is more common to find black diamonds by this treatment method than natural color black diamonds.
Over a century ago, scientists began experiments with irradiating diamonds which produced radioactive gemstones and color changes only near the surface of the material. The process has evolved since then (no longer radioactive!) and irradiation is another treatment method which can create wildly vibrant fancy colors in diamonds, not easily found in nature. However this treatment method is not as stable and when exposed to extreme heat (such as common jewelry repair work involving fire) irradiated color can change to a less desirable hue if the treatment is not disclosed.
Recent posts
Where to Find an Engagement Ring Like Taylor Swift’s
Let's break down the design and show you how to recreate the look!
Why Diamonds Are a Smart Investment
While traditional investment options like stocks, bonds, and real estate fluctuate with market trends, diamonds hold a distinct position as a tangible, portable, and enduring store of wealth.
The Smart Investment: Rising Gold Prices vs. Platinum Value
Gold has long been a symbol of luxury, but its soaring prices have changed the jewelry market landscape. With gold costs on the rise, the price difference between gold and platinum is shrinking. This shift means that for only a slightly higher investment you can enjoy the many advantages that platinum offers over gold.